Description
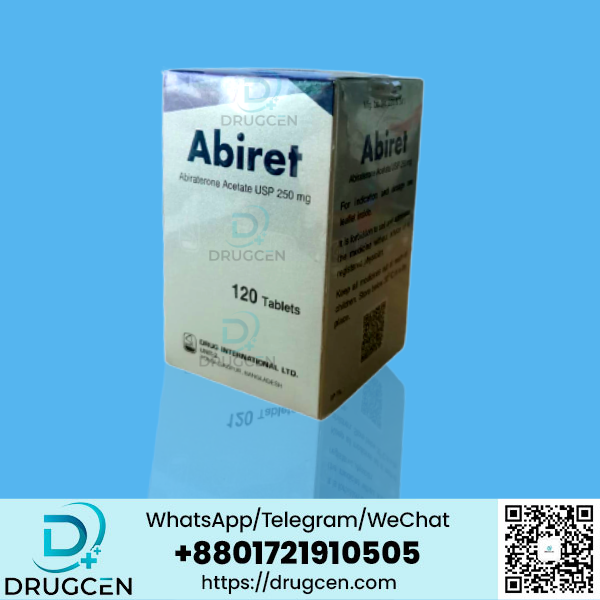
ABOUT ABIRET 250 Mg
The active ingredient in Abiret 250mg is Abiraterone Acetate USP 250mg. This medicine is produced and manufactured by Drug International LTD. This medicine is essential and effective medicine against prostate cancer. Abiret 250mg works by blocking the production of androgens in the body. It reduces the activity of the enzyme 17 beta-hydroxylase 20-lyase (CYP17). This enzyme is essential for androgen biosynthesis, so it is highly expressed in testicular, adrenal, and prostatic tumour tissues. This treatment is recommended for patients with advanced castration-resistant prostate cancer (CRPC) or castration-sensitive prostate cancer (CSPC) with a high risk of metastasis.
DOSAGE
Patients with advanced CRPC are typically prescribed four tablets once daily in addition to two 5mg oral doses of Prednisone. Metastatic high-risk CSPC patients, on the other hand, are advised to take four 250-mg tablets at once in addition to 5 mg of oral Prednisone once daily. You need to get some water and take the capsule whole. Make sure the capsule doesn’t get opened, broken, or chewed.
Side Effects
- Drowsiness.
- nauseousness.
- vomiting.
- low potassium levels.
- fever.
- cough.
- persistent cold and fluid retention.
STORAGE
Abiret 250mg needs to be kept cool (below 30 degrees Celsius), dark (away from light), and secure location (away from curious hands).




Reviews
There are no reviews yet.